"Piezo1, F-actin Remodeling, and Podocyte Survival and Regeneration"
https://doi.org/doi:10.1681/ASN.0000000697
https://pubmed.ncbi.nlm.nih.gov/40172977/
#Mechanical #Actin
#actin
"Dynamic Plasma Membrane Topography Linked With Arp2/3 Actin Network Induction During Cell Shape Change"
https://doi.org/doi:10.1002/bies.70004
https://pubmed.ncbi.nlm.nih.gov/40159841/
#Mechanical #Actin #Cell
"Regulation of plasma membrane tension through hydrostatic pressure and actin protrusion forces"
https://www.biorxiv.org/content/10.1101/2025.03.24.645030v1?rss=1 #Extracellular #Mechanical #Actin

"Time irreversibility, entropy production and effective temperature are independently regulated in the actin cortex of living cells"
https://arxiv.org/abs/2503.17016 #Physics.Bio-Ph #Cytoskeletal #Dynamics #Actin

"Live imaging in zebrafish reveals tissue-specific strategies for amoeboid migration"
https://doi.org/doi:10.1242/dev.204351
https://pubmed.ncbi.nlm.nih.gov/40114648/
#Mechanical #Actin
"Cytoskeletal repair: Zyxin relieves actin stress from the inside out"
https://doi.org/doi:10.1016/j.cub.2024.12.055
https://pubmed.ncbi.nlm.nih.gov/39999785/
#Cytoskeletal #Mechanical #Actin
"Actin crosslinking is required for force sensing at tricellular junctions"
https://www.biorxiv.org/content/10.1101/2025.02.21.639590v1?rss=1 #Mechanical #Force #Actin #Cell

"Mechanical Stretch Promotes the Migration of Mesenchymal Stem Cells via Piezo1/F-actin/YAP Axis"
https://doi.org/doi:10.1016/j.yexcr.2025.114461
https://pubmed.ncbi.nlm.nih.gov/39988125/
#Mechanical #Actin
"Calponin-Homology Domain of GAS2L1 Promotes Formation of Stress Fibers and Focal Adhesions"
https://doi.org/doi:10.1091/mbc.E24-10-0444
https://pubmed.ncbi.nlm.nih.gov/39969983/
#Mechanical #Actin
"Entanglement of vimentin shapes the microrheological response of suspended-like melanoma WM35 cells to oscillatory strains induced by different AFM probe geometries"
https://doi.org/doi:10.1016/j.bbagen.2025.130773
https://pubmed.ncbi.nlm.nih.gov/39954968/
#Mechanical #Vimentin #Actin
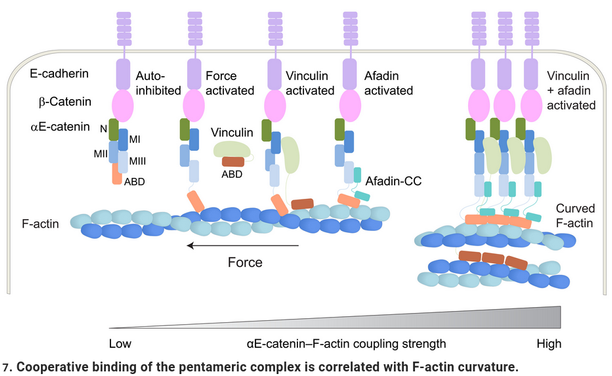
Seems #afadin (which likes #tricellular junctions) does have a role to stabilize #Ecadherin #cell-cell #adhesion and is sensitive to #actin curvature.
This may be of interest to #vertexModel fans and beyond (we have this paper on how turnover of #Ecadherin adhesion can modulate #cellIntercalation dynamics, https://journals.plos.org/ploscompbiol/article?id=10.1371/journal.pcbi.1009812)
"ATR-hippo drives force signaling to nuclear F-actin and links mechanotransduction to neurological disorders"
https://doi.org/doi:10.1126/sciadv.adr5683
https://pubmed.ncbi.nlm.nih.gov/39951537/
#Mechanical #Actin #Force #Cell
"Mouse scalp development requires Rac1 and SRF for the maintenance of mechanosensing mesenchyme"
https://www.biorxiv.org/content/10.1101/2025.02.11.637680v1?rss=1 #Mechanosensing #Mechanical #Actin

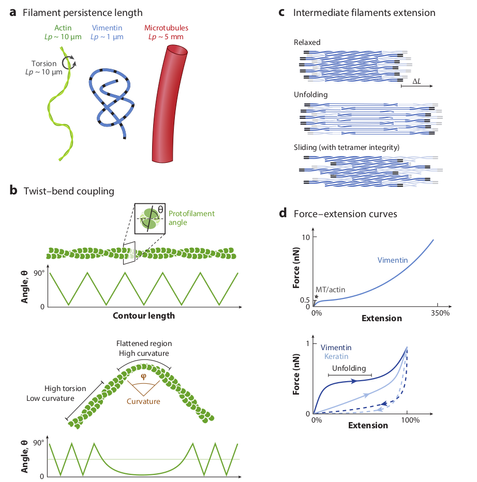
Interesting review !
"Far from being homogeneous beams, cytoskeletal filaments have complex mechanical properties, which are directly related to the specific structural arrangement of their subunits. They are also versatile, as the filaments’ mechanics and biochemistry are tightly coupled, and their
properties can vary with the cellular context."
"Modeling the prion protein-mediated transport of extracellular vesicles on the neuron surface"
https://arxiv.org/abs/2502.03610
#Physics.Bio-Ph #Actin

"Nuclear talin-1 provides a bridge between cell adhesion and gene expression"
https://doi.org/doi:10.1016/j.isci.2025.111745
https://pubmed.ncbi.nlm.nih.gov/39898029/
#Mechanical #Actin #Cell
"Arp2/3-mediated bidirectional actin assembly by SPIN90 dimers in metazoans"
https://www.biorxiv.org/content/10.1101/2025.01.31.635869v1?rss=1 #CellMigration #Actin

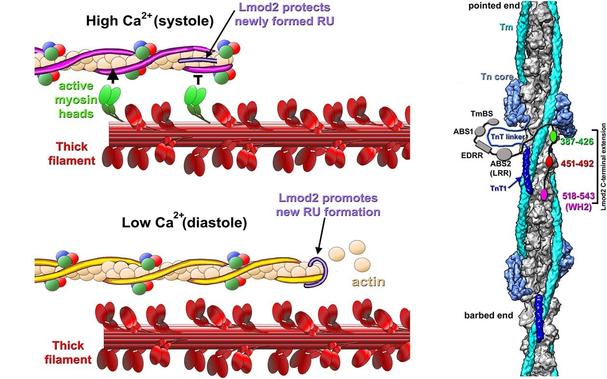
How is the length of actin-based thin filaments in the human #heart maintained? This study shows that #cardiac leiomodin regulates the length of thin filaments by protecting newly formed units during systole & promoting #actin polymerization during diastole #plosbiology https://plos.io/3CtG84I
"Cell Deformation Signatures along the Apical-Basal Axis: A 3D Continuum Mechanics Shell Model"
https://arxiv.org/abs/2501.17810
#Physics.Bio-Ph #Cond-Mat.Soft #Actin

"Intraluminal pressure triggers a rapid and persistent reinforcement of endothelial barriers"
https://www.biorxiv.org/content/10.1101/2025.01.22.634268v1?rss=1 #Mechanical #Actin